This tutorial demonstrates how to set up a Jukes and Cantor (1969, herafter named JC) model of nucleotide substitution, and then how to perform simulations and phylogenetic inference using this model. JC is the simplest model for describing nucleotide sequence evolution. It is a continuous time Markov chain (ctmc) model. In general ctmc models are fully characterized by their instantaneous-rate matrix:
\[Q = \begin{pmatrix} -\mu_A & \mu_{AC} & \mu_{AG} & \mu_{AT} \\ \mu_{CA} & -\mu_C & \mu_{CG} & \mu_{CT} \\ \mu_{GA} & \mu_{GC} & -\mu_C & \mu_{GT} \\ \mu_{TA} & \mu_{TC} & \mu_{TG} & -\mu_T \end{pmatrix} \mbox{ ,}\]where $\mu_{ij}$ represents the instantaneous rate of substitution from state $i$ to state $j$. The diagonal elements $\mu_i$ are the rates of not changing out of state $i$, equal to the sum of the elements in the corresponding row.
Each of the named substitution models (e.g., JC, TN92, HKY or GTR) has a uniquely defined instantaneous-rate matrix, $Q$.
In this tutorial you will perform phylogeny inference under the simplest model of DNA sequence evolution: JC. In the next tutorial, you will explore other models with names such as F81, HKY85, TN92, GTR, GTR+Gamma. In this tutorial, you will first simulate sequences using the JC model, and then perform phylogenetic inference from a sequence alignment. This inference will be based on the Markov chain Monte Carlo (MCMC) algorithm to estimate the phylogeny and other model parameters such as branch lengths. The estimated trees will be unrooted trees with independent branch-length parameters.
| Model | Reference | Function | Parameters |
|---|---|---|---|
| Jukes-Cantor | (Jukes and Cantor 1969) | fnJC | - |
| K80 (a.k.a. K2P) | (Kimura 1980) | fnK80 | $\kappa$ |
| Felsenstein-81 | (Felsenstein 1981) | fnF81 | $\pi$ |
| T92 | (Tamura 1992) | fnT92 | $\pi_{GC}$, $\kappa$ |
| HKY | (Hasegawa et al. 1985) | fnHKY | $\pi$, $\kappa$ |
| GTR | (Tavaré 1986) | fnGTR | $\pi$, $\epsilon$ |
Character Evolution under the Jukes-Cantor Substitution Model
Getting Started
This tutorial involves:
- setting up a Jukes-Cantor (JC) substitution model for an alignment of the cytochrome b subunit;
- simulating DNA sequence evolution;
- approximating the posterior probability of the tree topology and branch lengths using MCMC;
- summarizing the MCMC output by computing the maximum a posteriori tree.

We first consider the simplest substitution model described by Jukes and Cantor (1969). The instantaneous-rate matrix for the JC substitution model is defined as
\[Q_{JC} = \begin{pmatrix} {*} & \frac{1}{3} & \frac{1}{3} & \frac{1}{3} \\ \frac{1}{3} & {*} & \frac{1}{3} & \frac{1}{3} \\ \frac{1}{3} & \frac{1}{3} & {*} & \frac{1}{3} \\ \frac{1}{3} & \frac{1}{3} & \frac{1}{3} & {*} \end{pmatrix} \mbox{ ,}\]Simulating a DNA alignment
The files for this example analysis are provided for you (mcmc_JC.Rev).
If you download this file and place it in a directory called scripts inside your main tutorial directory,
you can easily execute this analysis using the source() function in the RevBayes console:
source("scripts/simulation_JC.Rev")
This simulation should be fast, and should have produced two files in the
folder data: simulatedSequences_1.fasta and simulatedSequences_2.fasta.
Below we are going to go through the script and explain what it does, step by step.
Loading the Data
The first thing the script does is to load a DNA alignment. Although this step is not necessary for simulating an alignment, here we use the empirical alignment just to get its number of sequences, its number of sites, and the sequence names. We will also compare the simulated sequences with the empirical sequences.
We load in the sequences using the readDiscreteCharacterData()
function.
data <- readDiscreteCharacterData("data/primates_and_galeopterus_cytb.nex")
Executing this line initializes the data matrix as a variable in the Rev environment. You can have a look at the list of variables in the environment as follows:
ls()
To report the current value of any variable, simply
type the variable name and press enter. For the data matrix, this
provides information about the alignment:
data
DNA character matrix with 23 taxa and 1141 characters
=====================================================
Origination: primates_and_galeopterus_cytb.nex
Number of taxa: 23
Number of included taxa: 23
Number of characters: 1141
Number of included characters: 1141
Datatype: DNA
Next we will specify some useful variables based on our dataset. The variable
data has member functions that we can use to retrieve information about the
dataset. These include, for example, the number of species and the taxa.
We will need that taxon information for setting up different parts of our model.
num_taxa <- data.ntaxa()
num_branches <- 2 * num_taxa - 3
taxa <- data.taxa()
num_sites <- data.nchar()
Here, we are defining constants of our model. In RevBayes, we define constants using “<-“. We will see later that we have two other assignment operators, for the random and deterministic variables of the model.
With the data loaded, we can now proceed to specifying the model.
Setting up the Graphical Model
Simulating DNA sequences with an unrooted tree under the JC model requires specification of two main components: (1) the and (2) the .
Jukes-Cantor Substitution Model
A given substitution model is defined by its corresponding
instantaneous-rate matrix, $Q$. The Jukes-Cantor substitution model does
not have any free parameters (as the substitution rates are all assumed
to be equal), so we can define it as a constant variable. The function
fnJC(n) will create an instantaneous-rate matrix for a character with
$n$ states. Since we use DNA data here, we create a 4x4
instantaneous-rate matrix:
Q <- fnJC(4)
You can see the rates of the $Q$ matrix by typing
Q
[ [ -1.0000, 0.3333, 0.3333, 0.3333 ] ,
0.3333, -1.0000, 0.3333, 0.3333 ] ,
0.3333, 0.3333, -1.0000, 0.3333 ] ,
0.3333, 0.3333, 0.3333, -1.0000 ] ]
As you can see, all substitution rates are equal.
Tree Topology and Branch Lengths
The tree topology and branch lengths are unknown (we want to estimate them). In Bayesian inference, we formalize this by defining them as random variables, with a prior probability distribution. In the graphical representation of our model, random variables are always shown by plain circles. They are also called stochastic nodes of our phylogenetic model. In , the tree topology is denoted $\tau$ and the length of the branch leading to node $i$ is $bl_i$.
We will assume that all possible labeled, unrooted tree topologies have equal probability a priori.
This is the dnUniformTopology() distribution in RevBayes.
In RevBayes it is advisable to specify the outgroup for your study system.
Here, we will use Galeopterus, which is the sister group to all other primates.
Specify the topology stochastic node by passing in the list of taxa and the outgroup
to the dnUniformTopology() distribution:
out_group = clade("Galeopterus_variegatus")
topology ~ dnUniformTopology(taxa, outgroup=out_group)
See how we define a new random variable (or a new stochastic node) of our model: by using the stochastic operator ‘~’. Thus, it is different from constants, for which we used ‘<-‘.
Next we have to create a stochastic node representing the length of each of the
$2N - 3$ branches in our tree (where $N=$ n_species). We can do this using a
for loop. In this loop, we can create each of the branch-length nodes.
Each branch length is a random variable, and we have to define a prior for each of them. Note that the branch lengths are measured in number of substitutions per site. Usually, these numbers are relatively small (of the order of $0.01$ or $0.1$). Usually, in phylogenetics, we use an exponential prior of mean $0.1$ for branch lengths. Equivalently, we use an exponential of rate 10 (the rate of an exponential distribution is the inverse of the mean).
Altogether, you can copy this entire block of Revcode into the console:
for (i in 1:num_branches) {
br_lens[i] ~ dnExponential(10.0)
}
See how we have defined a vector of branch lengths, just by defining all its components, using a for loop and using the indexation operator ‘[]’. Also, as above for the tree topology, we again use the stochastic operator ‘~’.
Finally, we can create a phylogram (a phylogeny in which the branch lengths are
proportional to the expected number of substitutions per site) by combining the
tree topology and branch lengths. We do this using the treeAssembly() function,
which applies the value of the $i^{th}$ member of the br_lens vector to the
branch leading to the $i^{th}$ node in topology:
psi := treeAssembly(topology, br_lens)
The psi variable is a deterministic node: its value is entirely determined by its two parents, topology and br_lengths (in the present case, it is just a container that puts together these two components of the model). Deterministic nodes are created using the ‘:=’ assignment operator. This is different from ‘<-‘, which was used for constants, because deterministic nodes will have to update their value when we will change the value of their parents.
Putting it All Together
We have fully specified all of the parameters of our phylogenetic
model—the tree topology with branch lengths, and the substitution model
that describes how the sequence data evolve over the tree with branch
lengths. Collectively, these parameters comprise a distribution called
the phylogenetic continuous-time Markov chain, and we use the
dnPhyloCTMC constructor function to create this node. This
distribution requires several input arguments:
- the
treewith branch lengths; - the instantaneous-rate matrix
Q; - the
typeof character data.
Build the random variable for the character data (sequence alignment).
seq ~ dnPhyloCTMC(tree=psi, Q=Q, type="DNA", nSites=num_sites)
Simulated data
Building the seq random variable automatically simulated sequences. You can
output them to a file:
writeFasta(filename="data/simulatedSequences_1.fasta", data=seq)
You can then view this alignment, for instance using seaview.
You can simulate another data set by “redrawing” the random variable:
seq.redraw()
And you can write the new sequences to another file:
writeFasta(filename="data/simulatedSequences_2.fasta", data=seq)
- Are they different from the first ones?
It is always important to evaluate the realism of simulations. In our case, we can compare the simulated alignment to the empirical alignment. The sequence data objects (“AbstractHomologousDiscreteCharacterData”) have lots of functions that we can use for this purpose:
data.methods()
Let’s use some of them to compare the empirical data to the simulated data set:
print("Mean GC content of the empirical data: " + data.meanGcContent())
print("Mean GC content of the simulated data: " + seq.meanGcContent())
print("Variance of the GC content of the empirical data: " + data.varGcContent())
print("Variance of the GC content of the simulated data: " + seq.varGcContent())
- Can you think of other statistics that might be interesting to compute to compare the empirical and the simulated data sets?
Performing inference with the JC model and the MCMC algorithm
In the first part of this tutorial, we saw how to simulate a DNA alignment using the JC model. The second part aims at performing inference under the JC model.
The inference script is called scripts/mcmc_JC.Rev.
You can run it in its entirety as follows:
source("scripts/mcmc_JC.Rev")
If everything loaded properly, then you should see the program initiate the Markov chain Monte Carlo analysis that estimates the posterior distribution. If you continue to let this run, then you will see it output the states of the Markov chain once the MCMC analysis begins.
Ultimately, this is how you will execute most analyses in RevBayes, with the full
specification of the model and analyses contained in the sourced files.
You could easily run this entire analysis on your own data by substituting your
data file name for that in the model-specification file. However, it is important
to understand the components of the model to be able to take full advantage of
the flexibility and richness of RevBayes. Furthermore, without inspecting the
Rev scripts sourced in mcmc_JC.Rev, you may end up inadvertently performing
inappropriate analyses on your dataset, which would be a waste of your time and
CPU cycles. The next steps will walk you through the full specification of the
model and MCMC analyses.
Additional elements in the inference script
Overall, the inference script is very similar to the simulation script. For instance, the structure of the model is the same. However, to perform inference, additional elements have to be included. In particular, the model that we were using for simulation has to be conditioned upon observed data, the sequences at the tips. Secondly, the whole model has to be assembled into a model variable, upon which MCMC sampling will be able to operate. Thirdly, moves need to be specified for each stochastic variable, so that we can obtain posterior distributions. Recall that moves are algorithms used to propose new parameter values during the MCMC simulation. Fourthly, we may want to specify monitors to record parameter values as the MCMC algorithm proceeds. Monitors print the values of model parameters to the screen and/or log files during the MCMC analysis.
Conditioning the model on observed data
The PhyloCTMC model is able to produce sequence alignments, given parameter
variables. We can attach our sequence data to the tip nodes in the tree:
seq.clamp(data)
Note that although we assume that our sequence data are random variables—they are realizations of our phylogenetic model—for the purposes of inference, we assume that the sequence data are “clamped” to their observed values. When this function is called, RevBayes sets each of the stochastic nodes representing the tips of the tree to the corresponding nucleotide sequence in the alignment. This essentially tells the program that we have observed data for the sequences at the tips: we are conditioning the model on these data.
Assembling the constant, deterministic and stochastic variables into a model
We wrap the entire model in a single object to provide convenient access to the
Directed Acyclic Graph. To do this, we only need to give the model() function a single
node of the model. Starting from this node, the model() function can find all of the other
nodes by following the arrows in the graphical model:
mymodel = model(Q)
Now we have specified a simple phylogenetic model—each parameter of
the model will be estimated from every site in our alignment. If we
inspect the contents of mymodel we can review all of the nodes in the
DAG:
mymodel
Adding moves to the script
For each stochastic variable of the model, we need to specify a move. If no move is specified for a stochastic variable, this variable will stay constant throughout the entire algorithm.
First we create a vector to store all the moves that will be used to sample from the posterior distribution:
moves = VectorMoves()
You may have noticed that we used the = operator to create the move index.
This simply means that the variable is not part of the model: it is not used to
compute the probability of a set of parameter values. Instead, it is a part
of the MCMC algorithm.
You will later see that we use this operator for other variables that are not
part of the model, e.g., when we create moves and monitors.
For instance, we specify a move for the tree topology.
moves.append( mvNNI(topology, weight=3) )
Some types of stochastic nodes, and the tree topology in particular, can be
updated by a number of alternative moves.
Different moves may explore parameter space in different ways,
and it is possible to use multiple different moves for a given parameter to improve mixing
(i.e. the efficiency of the MCMC simulation).
In the case of our unrooted tree topology, for example, we can use both a
nearest-neighbor interchange move (mvNNI in the Rev language) and a subtree-prune and regraft move (mvSPR in the Rev language, currently commented in the script).
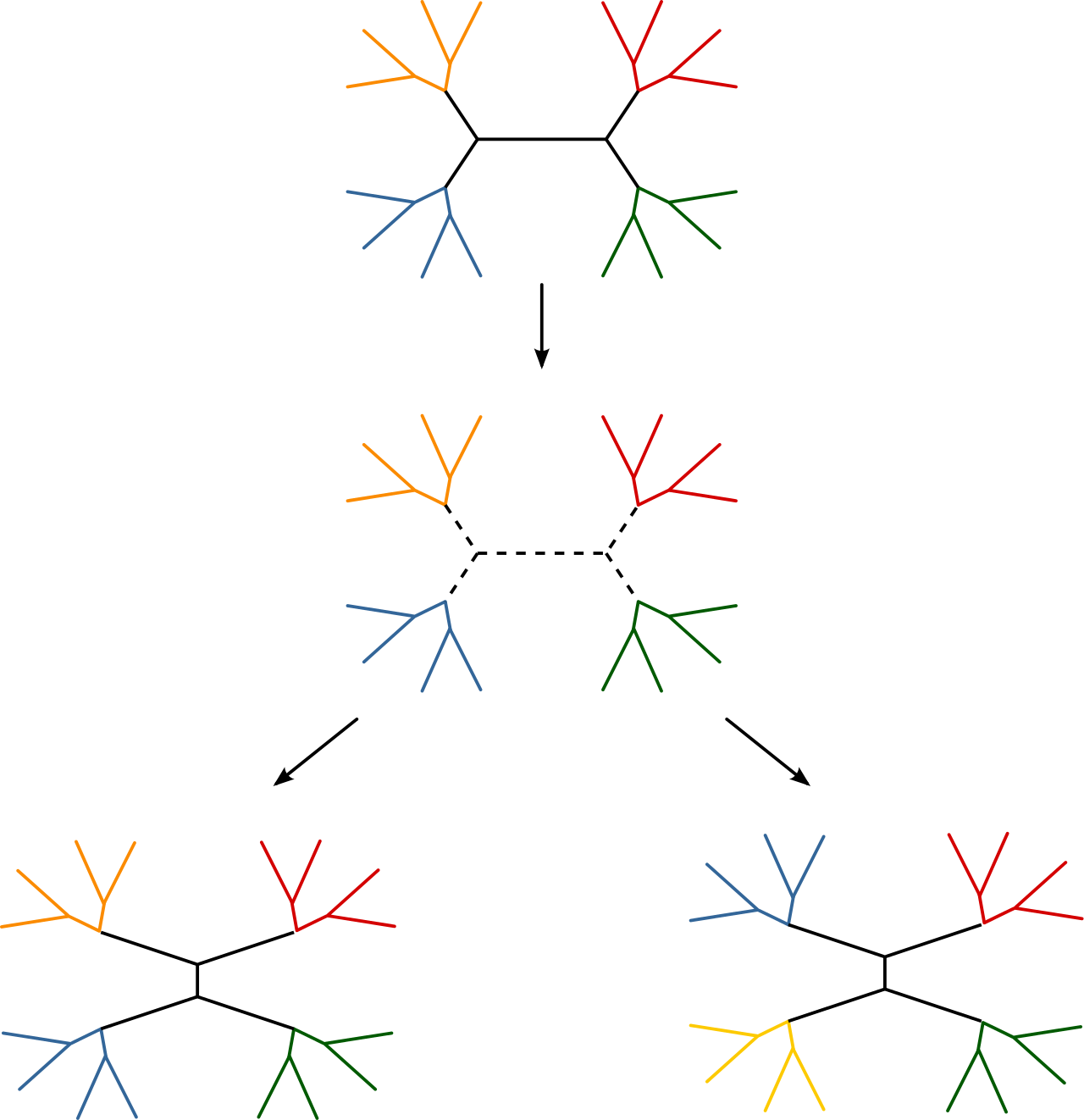

These moves do not have tuning parameters associated with them, thus we only need
to pass in the topology node and proposal weight.
moves.append( mvSPR(topology, weight=3) )
The weight specifies how often the move will be applied either on average per iteration or relative to all other moves. Have a look at the MCMC tutorials for more details about moves and MCMC strategies.
The other stochastic variables in the model are branch lengths. They need their own moves:
for (i in 1:num_branches) {
moves.append( mvScale(bl[i]) )
}
Here each branch length is associated to a mvScale move that basically multiplies
the current value of a branch length by a real positive random number.
- What type of stochastic variable is particularly appropriate for the
mvScalemove?
Specifying Monitors and Output Files
During the MCMC analysis, we can decide to keep track of all the sampled parameters,
but also of various statistics that we think may be of interest. Here, we have
decided to keep track of the tree length, i.e. the sum of all branch lengths.
To this end, we add a variable TL that will compute this sum. Accordingly, the tree
length can be computed using the sum() function, which calculates the sum of
any vector of values.
TL := sum(br_lens)
- Why are we using “:=” here?
For our MCMC analysis, we need to set up a vector of monitors to
record the states of our Markov chain. The monitor functions are all
called mn\*, where \* is the wild-card representing the monitor type.
First, we will initialize the model monitor using the mnModel
function. This creates a new monitor variable that will output the
states for all model parameters when passed into a MCMC function.
monitors = VectorMonitors()
monitors.append( mnModel(filename="output/primates_cytb_JC.log", printgen=10) )
The mnFile monitor will record the states for only the parameters
passed in as arguments. We use this monitor to specify the output for
our sampled phylograms.
monitors.append( mnFile(filename="output/primates_cytb_JC.trees", printgen=10, psi) )
Finally, we create a screen monitor that will report the states of
specified variables to the screen with mnScreen:
monitors.append( mnScreen(printgen=100, TL) )
This monitor mostly helps us to see the progress of the MCMC run.
Initializing and Running the MCMC Simulation
With a fully specified model, a set of monitors, and a set of moves, we
can now set up the MCMC algorithm that will sample parameter values in
proportion to their posterior probability. The mcmc() function will
create our MCMC object:
mymcmc = mcmc(mymodel, monitors, moves)
Now, run the MCMC:
mymcmc.run(generations=2500)
Here we set a low number of iterations. In general it is recommended to check for convergence; here it is possible that 2500 iterations may not be enough.
When the analysis is complete, you will have the files produced by the monitors in your output directory.
Saving and restarting analyses
MCMC analyses can take a long time to converge, and it is usually difficult to predict how many generations will be needed to obtain results. In addition, many analyses are run on computer clusters with time limits, and so may be stopped by the cluster partway through. For all of these reasons, it is useful to save the state of the chain regularly through the analysis.
mymcmc.run(generations=100000000, checkpointInterval=100, checkpointFile="output/primates_cytb_JC.state")The
checkpointIntervalandcheckpointFileinputs specify respectively how often, and to which file, the chain should be saved. Three different files will be used for storing the state: one with no extension, one with extension_mcmc, and one with extension_moves. When multiple independent runs are specified, they will automatically be saved in separate files (with extensions_run_1,_run_2, etc.).Restarting the chain from a previous run is done by adding this line:
mymcmc.initializeFromCheckpoint("output/primates_cytb_JC.state")before calling the function
mcmc.run(). The file name should match what was given ascheckpointFilewhen running the previous analysis. NB: Note that this line will create an error if the state file does not exist yet, and so should be commented out in the first run.The full MCMC block thus becomes:
mymcmc = mcmc(mymodel, monitors, moves, nruns=1) mymcmc.initializeFromCheckpoint("output/primates_cytb_JC.state") #comment this out for the first run mymcmc.run(generations=100000000, checkpointInterval=100, checkpointFile="output/primates_cytb_JC.state")
Summarizing MCMC Samples
Methods for visualizing the marginal densities of parameter values are not currently available in RevBayes itself. Thus, it is important to use programs like Tracer (Rambaut and Drummond 2011) to evaluate mixing and non-convergence.
Look at the file called output/primates_cytb_JC.log in Tracer.
There you see the posterior distribution of the continuous parameters,
e.g., the tree length variable TL.


It is always important to carefully assess the MCMC samples for the various parameters in your analysis. You can read more about MCMC tuning and evaluating and improving mixing in the other tutorials on the RevBayes website.
Analyzing the inferred phylogeny
We are interested in the phylogenetic relationship of the Tarsiers. Therefore, we need to summarize the trees sampled from the posterior distribution. RevBayes can summarize the sampled trees by reading in the tree-trace file:
treetrace = readTreeTrace("output/primates_cytb_JC.trees", treetype="non-clock")
The mapTree() function will summarize the tree samples and write the
maximum a posteriori tree to file:
map_tree = mapTree(treetrace,"output/primates_cytb_JC_MAP.tree")

Look at the file called output/primates_cytb_JC_MAP.tree in
FigTree. We show it in .
Note, you can query the posterior probability of a particular clade being monophyletic using the following command:
Lemuroidea <- clade("Cheirogaleus_major",
"Daubentonia_madagascariensis",
"Lemur_catta",
"Lepilemur_hubbardorum",
"Microcebus_murinus",
"Propithecus_coquereli",
"Varecia_variegata_variegata")
treetrace.cladeProbability( Lemuroidea )
Conclusion
During this tutorial, we used the simplest phylogenetic model to first simulate data, and then infer a phylogeny given an empirical data set. Sequence simulation is often used to test inference methods.
- What could you learn by performing MCMC inference on a simulated data set?
- Combine the scripts to perform inference on simulated data.
Sequence simulation is also often used to assess model adequacy, i.e. to ask if my model can reproduce the important features of the empirical data set.
- Based on a comparison of the empirical and simulated alignments, do you think that the JC model is adequate for this data set?
In the next tutorial, you will learn about more sophisticated models of sequence evolution.
- Felsenstein J. 1981. Evolutionary Trees from DNA Sequences: a Maximum Likelihood Approach. Journal of Molecular Evolution. 17:368–376. 10.1007/BF01734359
- Hasegawa M., Kishino H., Yano T. 1985. Dating of the Human-Ape Splitting by a molecular Clock of Mitochondrial DNA. Journal of Molecular Evolution. 22:160–174. 10.1007/BF02101694
- Höhna S., Heath T.A., Boussau B., Landis M.J., Ronquist F., Huelsenbeck J.P. 2014. Probabilistic Graphical Model Representation in Phylogenetics. Systematic Biology. 63:753–771. 10.1093/sysbio/syu039
- Jukes T.H., Cantor C.R. 1969. Evolution of Protein Molecules. Mammalian Protein Metabolism. 3:21–132. 10.1016/B978-1-4832-3211-9.50009-7
- Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 16:111–120.
- Rambaut A., Drummond A.J. 2011. Tracer v1.5. . http://tree.bio.ed.ac.uk/software/tracer/
- Tamura K. 1992. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+ C-content biases. Molecular Biology and Evolution. 9:678–687.
- Tavaré S. 1986. Some Probabilistic and Statistical Problems in the Analysis of DNA Sequences. Some Mathematical Questions in Biology: DNA Sequence Analysis. 17:57–86.